Nutritional Supplement Probiotic Viability
Overview
OFD’s LyoLock process improves probiotic viability.
A leader in the probiotic nutritional supplement space recently approached us and asked if we would be willing to participate in a viability comparison study. They had two different probiotic strains (4100 and 4500) they wanted us to process, using our expertise to optimize maximum shelf stability, which they would then compare against the same probiotic strains processed by a competitor freeze-drying service.
We felt confident that we could leverage our LyoLock process and utilize our expertise in the field of microbial stabilization to retain higher levels of time zero viability through an accelerated shelf life study. We welcomed the opportunity to collaborate on defining a testing plan that would cover all of the critical process steps necessary to stabilize these products.
Approach
Our Technical Services Team sat down with their Technical Services Team and defined a plan to achieve optimal retention of viability based upon their unique inputs and treatment of their products, coupled with specialized applications throughout our process and packaging services. Considerations included fermentation and concentration method, along with their proprietary cryopreservative formulation. We also leveraged our experience with the military to identify a package that contributed to longer retention of probiotic viability.
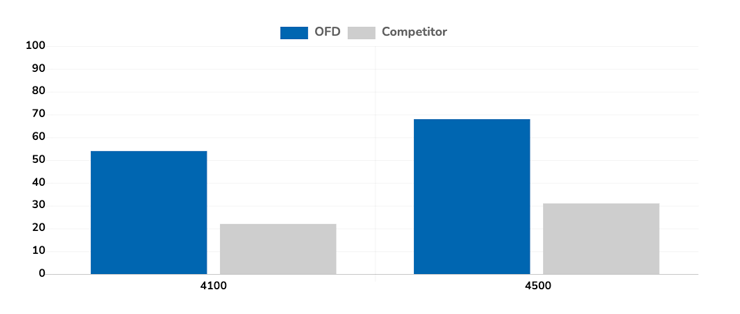
Outcome
The results, as seen in the attached graph, indicate that we were able to successfully apply our LyoLock process to achieve a higher relative percentage survival rate for both probiotic strains versus the competitive freeze-drying option. As a result of this study Oregon Freeze Dry won the business and continues to support this leading probiotic nutritional supplement company in the marketplace.
Related Case Studies
Let’s Win Together
For over 60 years OFD has set the standard of excellence in Lyophilization. We’re ready to apply our experience and unique manufacturing capabilities to find a successful, scalable solution to address your unique preservation challenge.